Download M Pharm Regulatory Affairs 1st Semester Syllabus – Master of Pharmacy in Regulatory Affairs is a specialized post-graduate program that focuses on the regulatory requirements for the development and approval of drugs, medical devices, and other healthcare products. The course is designed to provide students with the knowledge and skills needed to navigate the complex regulatory environment governing the pharmaceutical industry.
Download M Pharm Regulatory Affairs 1st Semester Syllabus
M Pharmacy Pharmaceutical Regulatory Affairs 1st semester is designed to provide a overview on the regulatory requirement’s require for marketing authorization of pharmaceuticals, medical devices, IVDs, cosmetics and nutraceuticals. You can download the PDF file of M Pharmacy (RA) 1st semester syllabus (as per PCI) from the below given “Download” button.
The M Pharmacy Regulatory Affairs 1st semester syllabus consists of five subjects that cover a wide range of regulatory topics. Let’s take a closer look at each subject:
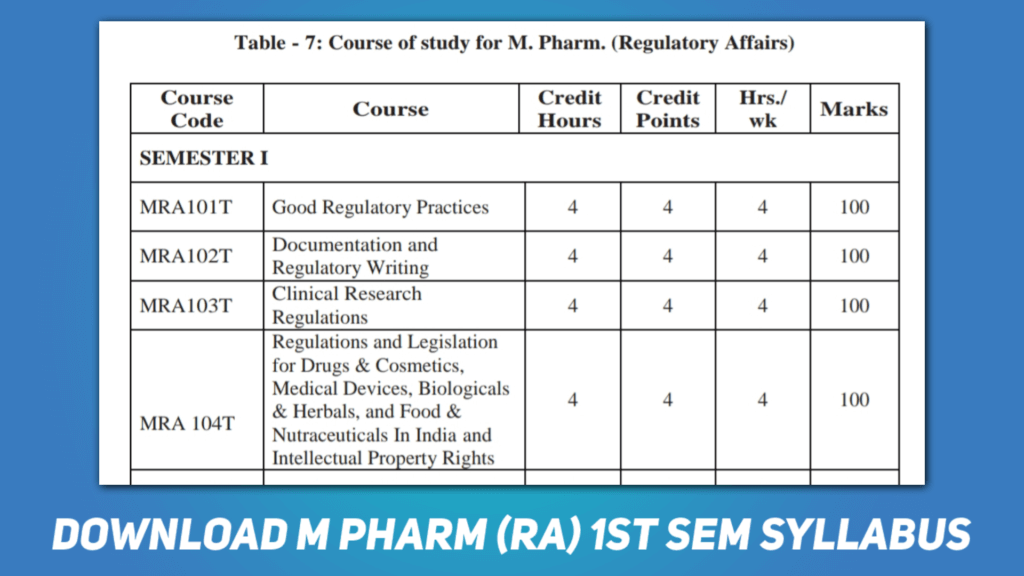
Good Regulatory Affairs
This subject covers the basics of regulatory affairs and the various regulatory bodies involved in drug development and approval. Students will learn about the different phases of drug development, clinical trials, and the regulatory requirements for marketing authorization. The course also covers topics such as pharmacovigilance, quality control, and post-marketing surveillance.
Related Post: Post
Document and Regulatory Writing
In this subject, students will learn how to write regulatory documents such as clinical study reports, regulatory submissions, and labeling documents. They will also learn about the guidelines and standards for regulatory writing, and how to communicate scientific information effectively to regulatory authorities.
Clinical Research and Regulations
This subject focuses on the regulations governing clinical trials and the ethical considerations involved in conducting clinical research. Students will learn about the various stages of clinical trials, from preclinical studies to post-marketing surveillance. They will also learn about the regulations governing the use of human subjects in clinical trials, and the ethical principles that guide clinical research.
Regulations and Legislation in India
This subject provides an overview of the regulatory landscape in India, including the regulatory bodies involved in drug development and approval. Students will learn about the various laws and regulations governing the pharmaceutical industry in India, including the Drugs and Cosmetics Act, the Pharmacy Act, and the Clinical Establishments Act.
Regulatory Affairs Practical 1
This subject provides students with hands-on experience in regulatory affairs through a practical project. Students will work on a regulatory project, such as preparing a regulatory submission, conducting a regulatory review, or developing a pharmacovigilance plan. They will also learn about project management and communication skills, which are essential for success in regulatory affairs.
Conclusion
In conclusion, the M Pharmacy (Regulatory Affairs) 1st semester syllabus covers a wide range of regulatory topics, from the basics of regulatory affairs to the regulations governing clinical trials and the pharmaceutical industry in India. The course provides students with the knowledge and skills needed to succeed in regulatory affairs and contribute to the development and approval of safe and effective healthcare products.

0 Comments