Download Good Regulatory Practices Unit 1 Notes – Regulatory compliance is an essential aspect of the pharmaceutical and biotech industries, ensuring that the products produced are safe and effective for use. Good regulatory practices (GRPs) are a set of guidelines and standards that regulate the manufacturing and distribution of pharmaceutical products. In this blog post, we will discuss the importance of GRPs and provide information on where to download Unit 1 notes that cover several key GRP guidelines.
Good Regulatory Practices Unit 1 Notes
Good Regulatory Practices (GRPs) are a set of principles and guidelines that pharmaceutical companies must follow to ensure that their products meet the required standards of quality, safety, and efficacy. Adhering to GRPs is critical to the success of the pharmaceutical industry, as it helps to ensure that products are manufactured and distributed in a manner that is consistent with regulatory requirements.

M Pharm RA Unit 1 Notes on GRPs
Good Regulatory Practices Unit 1 notes on GRPs cover a range of important guidelines and standards that pharmaceutical companies must follow. These notes include information on Current Good Manufacturing Practices (cGMP), US cGMP Part 210 and Part 211, EC Principles of GMP (Directive 91/356/EEC) Article 6 to Article 14, GAMP-5, and GHTF guidance document.
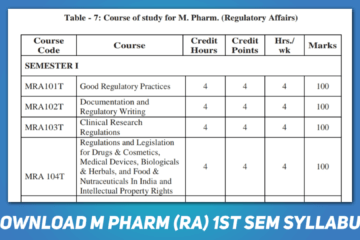
Related Article: Download M Pharm Regulatory Affairs 1st Semester Syllabus
Download M Pharm Regulatory Affairs Notes
You can download Good Regulatory Practices Unit 1 Notes by clicking on the download button.
Current Good Manufacturing Practices (cGMP) is a set of guidelines established by the United States Food and Drug Administration (FDA) that outlines the requirements for manufacturing, testing, and quality control of pharmaceutical products. The cGMP guidelines are designed to ensure that products are manufactured consistently and meet the required standards of quality and safety.
Notes on US cGMP Part 210 and Part 211
US cGMP Part 210 and Part 211 are specific regulations under the cGMP guidelines that outline the requirements for manufacturing and testing of pharmaceutical products. These regulations cover a range of topics, including facilities and equipment, quality control, production and process controls, packaging and labeling, and laboratory controls.
EC Principles of GMP (Directive 91/356/EEC) Article 6 to Article 14
EC Principles of GMP (Directive 91/356/EEC) Article 6 to Article 14 is a set of guidelines established by the European Union (EU) that outlines the requirements for manufacturing, testing, and quality control of pharmaceutical products. These guidelines are designed to ensure that products are manufactured consistently and meet the required standards of quality and safety.
GAMP-5 – Download Good Regulatory Practices Unit 1 Notes
GAMP-5 is a set of guidelines established by the International Society for Pharmaceutical Engineering (ISPE) that outlines the requirements for computerized systems used in the pharmaceutical industry. The guidelines cover a range of topics, including risk management, validation, and documentation.
Global Harmonization Task Force (GHTF)
The GHTF guidance document is a set of guidelines established by the Global Harmonization Task Force (GHTF) that outlines the requirements for medical device manufacturers. These guidelines are designed to ensure that medical devices are safe and effective for use.
Conclusion
Download Good Regulatory Practices Unit 1 Notes prepared by the student on M Pharm 1st Semester student. In conclusion, adherence to Good Regulatory Practices is essential for the pharmaceutical industry to ensure that the products produced are safe and effective for use.
Unit 1 notes on GRPs cover a range of important guidelines and standards that pharmaceutical companies must follow, including Current Good Manufacturing Practices, US cGMP Part 210 and Part 211, EC Principles of GMP (Directive 91/356/EEC) Article 6 to Article 14, GAMP-5, and GHTF guidance document. To download these notes, you can visit various online platforms that offer them for free or for a fee.
0 Comments